Reprinted from Chapter 28 of Bonica's Managment of Pain, Third Edition, John D. Loeser, MD, et al, Linnincott Williams & Wilkins
Myofascial pain syndromes are a large and diverse group of painful conditions that occur in the musculoskeletal system. They affect muscles and their connective tissue attachments in any part of the body, and are therefore customarily named according to the location of the painful part (e.g., lateral epi-condylitis, Achilles tendonitis, frozen shoulder, bicipital tendonitis, and even low back pain) (Table 28-1). They are puzzling because they seem to arise and persist in the absence of any detectable injury or inflammation. Myofascial pain syndromes are often difficult to treat because medications and the commonly available physical therapies give only temporary relief. Innumerable patients, therefore, wander from provider to provider in a vain search for relief.
The term myofascial pain syndrome is presently used in a vague and indeterminate way to denote any regional musculoskeletal pain syndrome without regard to its source or cause. However, careful examination of these syndromes often reveals them to be the effects of neuropathy appearing in the musculoskeletal system. The initial and underlying problem is malfunction of the peripheral nervous system, and pain is just one possible although not inevitable downstream product of the neuropathy. The key to successful management of this important and widespread category of chronic pain is to understand neuropathy, how it can cause pain, and recognize it in its many guises.

Medical diagnosis traditionally presumes that pain is a signal of tissue injury conveyed to the central nervous system via a healthy nervous system. The definition of pain, as given by the International Association for the Study of Pain, underscores this: "an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described by the patient in terms of such damage" (see Chapter 2). [AU: 1] The traditional model, however, fails to explain many pain syndromes associated with damage to the peripheral nerve, or to the pathways coursing through the dorsal horn and spinal cord. Although pain may be linked causally to tissue injury, it is not necessarily so. Injury does not always generate pain, nor does pain always signal injury. Persistent pain can occur in the presence of the following conditions: (a) ongoing nociception (eg, an unhealed fracture), or persistent inflammation (eg, rheumatoid arthritis). One way in which inflammation is triggered is in response to lipopolysaccharides that are released from the cell walls of many bacteria - which activate the immune system's macrophages. These cells in turn, release "alarm" molecules which, together with algogenic substances such as bradykinin, serotonin, histamine, H+, K+, prostaglandins, leukotrines, nerve growth factors and neuropeptides, can cause pain. Two molecules, Cytokines and Tumour-necrosis factor [TNF], have powerful pro-inflammatory properties that can affect nearly all cell types and has a range of biological activities; (b) psychological factors such as somatization disorders, depression, or adverse operant learning processes; (c) abnormal functioning in the nervous system. Pain can arise from nonnoxious input, or from within the body when there is some functional disturbance in the nervous system (e.g., peripheral neuropathy). Neuropathic pain is generally used to refer to any acute or chronic pain syndrome in which the mechanism that sustains the pain is inferred to involve aberrant somatosensory processing in the peripheral nervous system or central nervous system. In neuropathic myofascial pain, structural factors exist as well, such as muscle shortening, degraded and weakened collagen, and trophic changes that contribute to the pain.
It is well accepted that pain can follow gross nerve injury, but the many and varied effects of dysfunction in the peripheral nervous system are recent concerns. I proposed the concept of neuropathic pain when it became evident from clinical observations and research carried out at the Workers' Compensation Board of British Columbia that pain is not always a signal of injury, but can be a product of abnormal nerve function (1).
In 1970, my examination of patients who had back pain, but no signs of injury, showed that those who were disabled for long periods had tenderness over muscle motor points in affected myotomes. Tender motor points, I found, are sensitive indicators of radicular involvement or irritation at the nerve root. Tender points differentiate a simple mechanical low back strain, which usually heals quickly, from one that is slow to improve (2). Next, a study of patients with tennis elbow showed that tender points at the elbow were secondary to cervical spondylosis and radiculopathy (i.e., neuropathy originating at the nerve root). Treating the neck, but not the elbow, was able to provide relief (3). A study of pain in the shoulder likewise implicated neuropathy at the cervical spine (4).
A pattern began to emerge: Patients who have pain, but no signs of injury, generally have sensory, motor, and autonomic manifestations of peripheral neuropathy. Peripheral neuropathy may be defined as a disease that causes disordered function in the peripheral nerve. Although sometimes associated with structural changes in the nerve, a neuropathic nerve can, deceptively, appear normal. It still conducts nerve impulses, synthesizes and releases transmitted substances, and evokes action potentials and muscle contraction.
In 1978, I presented a paper to the Royal College of Physicians and Surgeons of Canada reporting that following neuropathy and denervation, "many diverse pain syndromes of apparently unrelated causation may be attributed to supersensitive receptors (nociceptors) and hyperreactive control systems at internuncial pools." The concept of neuropathic pain is well accepted today, but at that time, not all physicians were familiar with peripheral neuropathy, and some vigorously denied its existence (Mersky H, personal communication). [AU: 2]
The following attributes generally are associated with neuropathic pain (5,6):
Pain when no ongoing tissue-damaging process exists.
Delay in onset after precipitating injurv. It generally takes approximately 5 days for supersensitivity to develop (7).
Dysesthesia, unpleasant burning or searing sensations, or deep, aching pain that is more common than dysesthetic pain in musculoskeletal pain syndromes.
Pain felt in a region of sensory deficit.
Neuralgic pain, paroxysmal brief shooting or stabbing pain.
Severe pain in response to a noxious stimulus (hyperalgesia).
Severe pain in response to a stimulus that is not normally noxious (allodynia).
Pronounced summation and afterreaction with repetitive stimuli.
Neuropathic pain is customarily perceived as beginning with peripheral sensitization. In peripheral sensitization, increased transduction sensitivity of nociceptors is associated with alteration of ionic conductances in the peripheral terminal (see Chapter 3). [AU: 3] Sensitization can occur following tissue inflammation or damage to a peripheral nerve. Inflammatory cells also produce growth factors and cytokines that contribute to the increased sensitivity of nociceptors. However, damage to the peripheral nerve is most commonly caused by spondylosis at root level (i.e., radiculopathic pain) when all fibers of the peripheral nerve can be damaged and can lead to any or all of the following effects:
Motor: Muscle shortening is the most significant feature of radiculopathy and pain is caused by increased sensitivity as well as the secondary, mechanical effects of muscle shortening.
Autonomic: Increased vasoconstriction; hyperhidrosis; trophedema; and causalgic pain, reflex sympathetic dystrophy (RSD).
Sensory:
Trophic: Dermatomal hair loss; collagen degradation and frailty leading to enthesopathic tendons.
This title, Complex Regional Pain Syndrome (CRPS), is now introduced to cover the painful syndromes formally described under the headings of "Reflex Sympathetic Dystrophy (RSD)" and "Causalgia". It is now recognized these syndromes begin with a nerve injury, but not all cases seem to have sympathetically maintained pain, and not all were dystrophic. Our radiculopathy model recognizes and explains the above effects or manifestations of neuropathy, which can be mixed and have variable presentations. We do not use the confusing and inane terms "Complex Regional Pain Syndrome, Type I (Reflex Sympathetic Dystrophy) (1 - 4)". Our model uses a specialized examination technique which detects vasoconstriction which is generally present, impairment of motor function, as well as trophic edema.
Treatment must be directed to the cause of the neuropathy. When neuropathy is relieved, the above changes, including vasoconstriction are resolved. Thus, RSD is another example of chronic soft tissue pain which is often incorrectly diagnosed and ineffectively treated.
Inflammation is clinically obvious, but minor damage to the nerve root is not. Early radiculopathy is universal, but it is usually unsuspected in prespondylosis when painless muscle shortening precedes peripheral sensitization. Damaged primary afferent fibers demonstrate three electrophysiologic features: (a) spontaneous activity; (b) exaggerated response to stimulus; and (c) sensitivity to catecholamines. These can be explained by a fundamental physiologic law.
AR (48), an educational assistant, complained of severe pain in the left foot from a running injury. Diagnosis RSD, treated with Gabapentin with no improvement. Arrived using crutches.
Physical findings in both back and lower leg. IMS treatment since July 14, 2003, to back and left leg. December 29, 2003, has no pain at rest and foot is rapidly improving, not using any walking aids.
This law is seldom cited to explain neuropathic pain; it deserves to be better known. It points out that the normal physiology and integrity of all innervated structures are dependent on the arrival of nerve impulses via the intact nerve to provide a regulatory or trophic effect. When this flow, which is probably a combination of axoplasmic flow and electrical input, is blocked, innervated structures are deprived of the trophic factor, which is vital for the control and maintenance of cellular function. A-trophic structures become highly irritable and develop abnormal sensitivity or supersensitivity according to Cannon and Rosenblueth's Law of Denervation (7): "When a unit is destroyed in a series of efferent neurons, an increased irritability to chemical agents develops in the isolated structure or structures, the effect being maximal in the part directly denervated."
All denervated structures develop supersensitivity (including skeletal muscle, smooth muscle, spinal neurons, sympathetic ganglia, adrenal glands, sweat glands, and brain cells). Cannon and Rosenblueth's original work was based on total denervation or decentralization for supersensitivity to develop; accordingly, they named the phenomenon denervation supersensitivity. But it is now known that physical interruption and total denervation are not necessary: Any circumstance that impedes the flow of motor impulses for a period of time can rob the effector organ of its excitatory input and cause disuse supersensitivity in that organ and, significantly, in associated spinal reflexes (8).
The importance of disuse supersensitivity cannot be overemphasized. When a nerve malfunctions, the structures it supplies become supersensitive and behave abnormally. These structures overreact to many forms of input, not only chemical, but physical inputs as well, including stretch and pressure. Supersensitive muscle cells can generate spontaneous electrical impulses that trigger false pain signals or provoke involuntary muscle activity (9). Supersensitive nerve fibers become receptive to chemical transmitters at every point along their length instead of at their terminals only. Sprouting may occur, and denervated nerves are prone to accept contacts from other types of nerves including autonomic and sensory nerve fibers (10). Short circuits are possible between sensory and autonomic (vasomotor) nerves and may contribute to complex regional pain syndrome.
Disuse supersensitivity is basic and universal, yet not at all well known or credited. The important role of supersensitive structures after neuropathy or denervation was previously neglected. Many diverse pain syndromes of apparently unknown causation may be attributed to the development of hypersensitive receptor organs and supersensitivity in pain sensory pathways. Instead of nociception, there can be severe pain in response to a noxious stimulus (hyperalgesia) or severe pain in response to a stimulus that is not normally noxious (allodynia).
It is not unusual for the flow of nerve impulses to be obstructed; peripheral neuropathy, often accompanied by partial denervation, is not exceptional in adults. Of the innumerable causes of nerve damage, such as trauma, metabolic, degenerative, toxic, and other conditions, chronic attrition from spondylosis (the structural disintegration and morphologic alterations that occur in the intervertebral disk, with pathoanatomic changes in surrounding structures) is by far the most common. The spinal nerve root, because of its vulnerable position, is notably prone to injury from pressure, stretch, angulation, and friction. Other causes of radiculopathy (i.e., neuropathy at the nerve root), such as arachnoiditis, neuroma, and intraspinal tumors, are much less common. Spondylosis increases with age; therefore, spondylotic pain is more common in middle-aged individuals who have accumulated an injury pool, an accumulation of repeated major and minor injuries to a segment leading to unresolved clinical residuals that may, or may not, produce pain (11).
Ordinarily, Spondylosis follows a gradual, relapsing, and
remitting course that is silent, unless and until, symptoms are precipitated by
an incident often so minor that it passes unnoticed by the patient. All
gradations of Spondylosis can exist, but early or incipient spondylotic changes,
even when unsuspected, can nevertheless irritate and upset function in the
segmental nerve. The emphasis on radiculopathy is not without reason:
With an acute injury to a healthy nerve, there is no prolonged discharge of pain
signals, whereas the same injury to a neuropathic nerve can cause a sustained
discharge (12). In other words, for pain to become a persistent symptom, the
affected fibers must be previously irritated or defective. That is why some
people develop severe pain after an apparently minor injury, and why that pain
can continue beyond a reasonable period.
The manifestations of neuropathic dysfunction are motor, sensory, and autonomic. In our studies, early and subtle signs of peripheral neuropathy were found in a significant number of young (under 30 years), apparently normal, and asymptomatic individuals (13). Brief and transient motor manifestations are the first to appear, and radiculopathy can occur without pain. Muscle shortening is an early and regular feature of radiculopathy, because large diameter nerve fibers at the nerve root -- axons of motoneurons and myelinated primary afferents (muscle proprioceptors) -- are the first to suffer physically. Painless, reversible, tight muscle knots can be felt in most individuals; not uncommonly, even in toddlers. Pain is not therefore a feature of radiculopathy unless nociceptive pathways are involved. Many neuropathies are pain free, such as sudomotor hyperactivity in hyperhidrosis, and muscle weakness in ventral root disease.
Ironically, neuropathy itself contributes to degenerative conditions (including Spondylosis). Neuropathy degrades the quality of collagen, causing it to have fewer cross-links; it is, therefore, frailer than normal collagen (14). The amount of collagen in soft and skeletal tissues is also reduced. Because collagen lends strength to ligament, tendon, cartilage, and bone, neuropathy can expedite degeneration in weight-bearing and activity-stressed parts of the body, which include the spine and joints, and become a source of pain. Enthesopathic thickening in a tendon is possibly a compensation for this weakness.
Interactions between peripheral and central mechanisms occur to produce postinjury hvpersensitivity and neuropathic pain. The spinal cord is not simply a passive conveyer of peripheral sensation to the brain: It can modify or amplify incoming signals. Central sensitization, a state of hyperexcitability of the dorsal horn neuron, can occur after damage to a peripheral nerve (e.g., irritation of a nerve root by Spondylosis) or after low-frequency repetitive C fiber nociceptor input (e.g., from peripheral tissue inflammation, as in arthritis). Central sensitization has several aspects: increased spontaneous activity of dorsal horn neurons, increased response to afferent input, expansion of receptive field size, reduction in threshold, and prolonged afterdischarges. Central sensitization leads to a cascade of molecular events, such as activation of the N-methyl-D-aspartate (NMDA) channel, increase in intracellular Ca2+, wind-up/wide dynamic range (WDR) neuron sensitization, and other phenomena (15-21).
Central sensitization may be maintained by ongoing primary afferent input, by altered central neural circuitry, or both. Woolf has identified four stimulus-processing states (22,23):
Normal state: In this state, low-intensity A-ß stimulation, such as touch, is perceived as innocuous, but high-intensity A-δ and C noxious stimulation are perceived as pain.
Suppressed state: High-intensity stimulation is not painful because of inhibition from segmental inhibition or descending inhibition from higher centers.
Sensitized state: Low-intensity stimulation is perceived as painful mechanical allodynia, and high-intensity stimulation, which is normally painful, leads to hyperalgesia.
Reorganized state: There may be structural changes and reorganization of the dorsal horn circuitry. Inappropriate synapses may form. A-ß fibers from layer 3 can sprout into layers 1 and 2, causing low-threshold afferent input to be misinterpreted as pain. A-ß afferents acquire the capacity, after inflammation, to produce central excitability, something they cannot normally do.
The clinical observation that pain radiates several segments
above and below the level of nociceptive stimulation may be explained by
dispersion of the primary afferent input through propiospinal connections in
adjacent layers 5 and 6 of the dorsal horn. This area also contains WDR neurons
(so called because they can encode a range of stimuli from light touch to
intense pain). The WDR receptive field is immense compared with that of the
primary afferent neuron; therefore, any increase in nociceptive stimulation can
lead to the recruitment of many more WDR neurons.
Perceived pain often outlasts the stimulus. In neuropathy, a brief discharge from A-δ or C fibers generates prolonged activity of WDR neurons because the normal inhibitory effects of A-ß fibers on A-d and C activity is lost.
Wind-up is a frequency-dependent phenomenon. Low-frequency (0.1 Hz) C fiber input gives a constant response from dorsal horn neurons. But frequencies greater than 0.5 Hz can give rise to hyperexcitability lasting for many minutes after the stimulus. In wind-up, C fibers release substance P, neurokinin A, and excitatory amino acids (glutamate and aspartate) onto dorsal horn neurons. There are two types of receptor at the dorsal horn, a neurokinin receptor and an NMDA receptor for amino acids. The binding of amino acids to the NMDA receptor depends on its prior activation by the binding of substance P to the neurokinin receptor. Thus, the release of substance P may lead to the recruitment of a second receptor type (NMDA) and an exaggerated response to further stimulation. The sensitized cell undergoes other biochemical changes, as indicated by the expression of the gene c-fos. Products of c-fos expression are involved in the regulation of neurotransmitter and nerve growth factor synthesis.
Diagnosing pain and dysfunction caused by radiculopathy depends almost entirely on the examiner's clinical experience and acumen. The history gives little assistance. Pain often arises spontaneously with no history of trauma, or else the degree of reported pain far exceeds that consistent with the injury. Laboratory and radiologic investigations are generally not helpful. Thermography reveals decreased skin temperature in affected dermatomes and this can be an indication of neuropathy, but does not necessarily signify pain. Radiculopathies are difficult to document with routine nerve conduction studies, which measure only the few fastest conducting and largest fibers and take no account of the majority of smaller fibers. In focal neuropathy, nerve conduction velocities remain within the wide range of normal values, but F-wave latency may be prolonged. Electromyographv is not specific either.
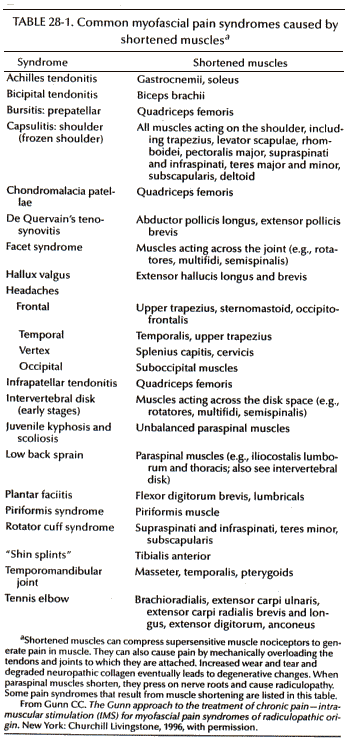
The physical signs of neuropathy are distinctive and different from the well-known ones of outright denervation, such as loss of sensation and reflexes. They are important to look for because they indicate early neural dysfunction for which no satisfactory laboratory or imaging test exists. A careful inspection for signs of motor, sensory, and autonomic (vasomotor, sudomotor, and pilomotor) dysfunction in the skin and affected muscles is necessary. Vasoconstriction differentiates neuropathic pain from inflammatory pain: In neuropathic pain, affected parts are perceptibly colder. There may be increased sudomotor activity and the pilomotor reflex is often hyperactive and visible in affected dermatomes as goose bumps (Fig. 28-1). There can be interaction between pain and autonomic phenomena. A stimulus such as chilling, which excites the pilomotor response, can precipitate pain; vice versa, pressure on a tender motor point can provoke the pilomotor and sudomotor reflexes.
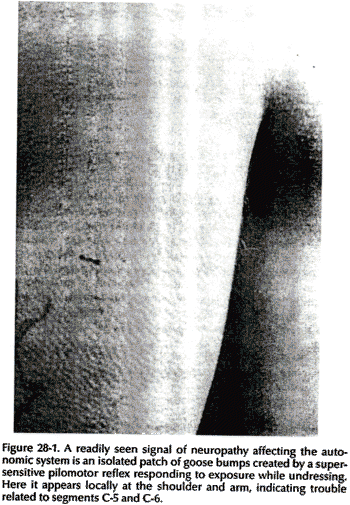
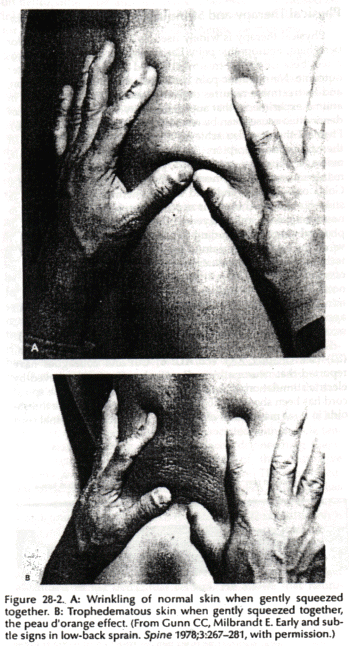
Increased permeability in blood vessels can lead to local subcutaneous tissue edema (neurogenic edema or trophedema). This can be seen as peau d'organge skin (Fig. 28-2) and confirmed by the match stick test. Trophedema is nonpitting to digital pressure, but when a blunt instrument such as the end of a match stick is used, the indentation produced is clear-cut and persists for many minutes (Fig. 28-3). This quick and simple test can demonstrate neuropathy earlier than electromvography. Trophic changes such as dermatomal hair loss may also accompany neuropathy.
Neuropathic changes are primarily in muscle. Even when symptoms appear to be in joints or tendons, signs in the muscles are the most consistent and relevant: increased muscle tone; tenderness over motor points; and taut and tender, palpable contracture bands and restricted joint range. Each constituent muscle must be palpated and its condition noted. Palpation requires detailed knowledge of anatomy, and clinical skill comes only with practice. Moreover, because manv paraspinal muscles are compound (e.g., the longissimus) and extend throughout most of the length of the vertebral column, the entire spine must be examined even when symptoms are localized to one region.
Muscle contracture is a fundamental feature of musculoskeletal pain (Fig. 28-4). Of all structures that can develop supersensitivity, the most widespread is striated muscle. Contracture can physically give rise to pain by its relentless pull on sensitive structures (24) (Fig. 28-5). Classic contracture refers to the evoked shortening of a muscle fiber in the absence of action potentials.
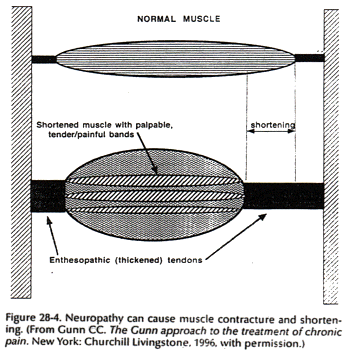
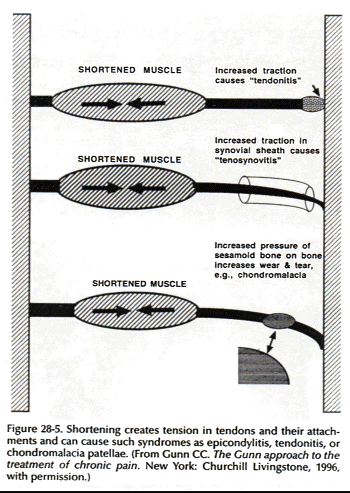
According to Cannon and Rosenblueth (7), skeletal muscle can become supersensitive in several ways: (a) by increased susceptibility; lessened stimuli, which do not have to exceed a threshold, can produce responses of normal amplitude; (b) by hyperexcitability; the threshold of the stimulating agent is lower than normal; (c) by superreactivity; the capacity of the muscle to respond is augmented; and (d) by superduration of response; the amplitude of response is unchanged but its time course is prolonged.
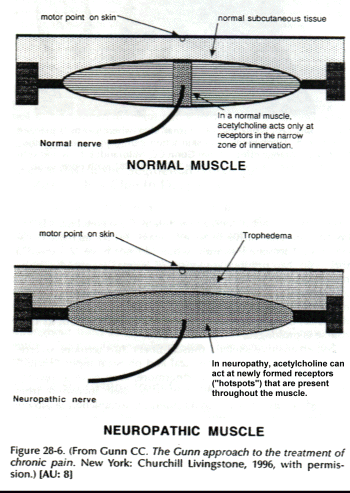
Supersensitive skeletal muscle fibers, furthermore, overreact to a wide variety of chemical and physical inputs, including stretch and pressure. Furthermore, they have a lowered threshold to acetylcholine, which is itself increased from reduced levels of acetylcholinesterase. Acetylcholine slowly depolarizes supersensitive muscle membrane, inducing an electromechanical coupling in which tension develops slowly without generating action potentials. In normal muscle, acetylcholine acts only at receptors that are situated in the narrow zone of innervation, but in neuropathy, it acts at newly formed extrajunctional receptors (hot spots) that appear throughout the muscle (Fig. 28-6).
A critical review of controlled clinical trials for peripheral neuropathic pain concluded that the pharmacologic management of neuropathic pain is difficult (25). Further trials are needed to establish the efficacy for all treatments currently in use. The review gave support to corticosteroids, which had long-term effectiveness, and limited support to tricyclic antide-pressants, intravenous and topical lidocaine, intravenous ketamine, carbamazepine, and topical aspirin. There was also limited support for oral, topical, and epidural clonidine and for subcutaneous ketamine. Data were contradictory for mexiletine, phenytoin, topical capsaicin, oral nonsteroidal anti-inflammatory medication, and intravenous morphine. Codeine, magnesium chloride, propanol, lorazepam, and intravenous phentolamine all failed to provide pain relief. There was also limited support for the effectiveness of topical dimethyl sulfoxide, epidural clonidine, and intravenous regional blocks with bretylium and ketanserin. Data were contradictory for intranasal calcitonin and intravenous phentolamine. Guanethidine, reserpine and droperidol IVRBs were ineffective. [AU: 4] No data were available to evaluate sympathetic ganglion blocks with local anesthetics. Regional and systemic adrenergic blockages gave limited success. Overall, there were no long-term data to support the effectiveness of any drug in treating this condition.
Physical therapy is widely used as a first-line treatment for peripheral neuropathic pain. Early physical treatment is advocated, because earlier treatment is said to correlate with better outcome. Neuropathic pain is a supersensitivity phenomenon, and its treatment requires desensitization. Lomo has shown in animal experiments that supersensitivity and other features of denervated muscle can be reversed by electric stimulation (26). Physical therapy also achieves its effect by stimulation. Local therapy excites receptors (in skin and muscle); for example, massage activates tactile and pressure receptors; exercise, manipulation, and dry needling stimulate muscle spindles and Golgi organs; heat and cold act on thermal receptors. These stimuli are sensed by their specific receptors, transduced into nerve impulses, and relayed to the dorsal horn. All forms of physical therapy, including dry needling, are effective only when the nerve to the painful part is still intact. A dry needling technique called intramuscular stimulation (see Chapter XX) is often effective. [Ed/AU: 5] In intramuscular stimulation, diagnosis, treatment, as well as progress during therapy are determined according to physical signs of neuropathy. The effective application of intramuscular stimulation therefore requires a sound background both in anatomy and neurophysiology (27).
Stimulation also can be applied directly to the spinal cord (28) (see Chapter XX). [Ed/AU: 6] Cui and colleagues have reported that neuropathic pain may be effectively relieved by electric stimulation of the spinal cord. Stimulation of the spinal cord has been shown to normalize withdrawal response thresholds in a rat model. The effect of stimulation of the spinal cord on neuropathic pain and allodynia is believed to be caused by inhibition of glutamate and aspartate release at NMDA receptor sites, and activation of local GABAergic mechanisms.
Spondylosis is, by far, the most common cause of radiculopathy, and treatment should be aimed at relieving the cause of impingement or entrapment of the nerve root. Local treatment (starting with simple measures such as massage, and, if necessary, escalating to more effective modalities such as dry needling) should be given to all tender and shortened muscles in the affected myotome(s), including paraspinal muscles. The outcome of treatment depends on the modality used and the skill of the therapist.
The fine, flexible, acupuncture needle used in intramuscular stimulation is a unique tool for finding and releasing contractures. Contracture is invisible to radiography, computed tomographic scans, or magnetic resonance imaging, and in deep muscles beyond the finger's reach. Deep contracture can only be discovered by probing with a needle. The needle transmits feedback information on the nature and consistency of the tissues it is penetrating. When penetrating normal muscle, it meets with little hindrance; when penetrating a contracture, there is firm resistance, and the needle is grasped by the muscle. This causes the patient to feel a peculiar, cramplike or grabbing sensation, which is referred to in acupuncture literature as the Deqi or Teh Ch'i response. The Deqi response is an important finding: it is a sign of muscle contracture and confirms the status of neuropathy.
Myofascial muscle pain is not merely dull and aching: It has a peculiar, cramplike quality that is associated with muscle tenderness and shortening. Any experienced dry-needling therapist or acupuncturist would be aware of this distinctive sensation produced by needling a contracture. The classic acupuncturist painstakingly differentiates between pain that has the Deqi response (therefore, the muscle is shortened and neuropathic), and pain that does not (nociceptive). This distinction is important because of the difference in the nature and treatment of the two pains. According to Fields, the strange quality of neuropathic pain probably results from disruption of the sensory apparatus so that a normal pattern of neural activity is no longer transmitted to the perceptual centers. He allows that neuropathic pain probably activates nociceptive neurons, because the message that gets through to the perceptual centers is clearly unpleasant, but he astutely notes that patients distinguish the peculiar sensations from normal pain sensations (29).
Chronic myofascial pain is not ordinary nociception: Deqi pain sensations are not normal because they are associated with receptors that sense muscle shortening (proprioceptors). The classic acupuncturist demonstrates this by the needle grasp occurring at the site of penetration when a neuropathic muscle is needled. Needling is usually pain free when an acupuncture needle enters a normal muscle, but when the needle pierces a shortened muscle, it produces a cramp, and the needle is observed to be firmly grasped by the shortened muscle. The intensity of the needle grasp parallels the degree of muscle shortening, and it gradually eases off during treatment as muscle shortening is released: Release frequently occurring in minutes. Because muscle pain eases concurrently with the release of the needle grasp, patients soon become aware of the importance of eliciting the Deqi sensation and releasing needle grasp during treatment.
Laboratory investigators presently pursue A-δ and C fiber nociceptive pathways but give little consideration to large-diameter fibers. However, Ma and Woolf have described a noteworthy phenomenon, progressive tactile hypersensitirity. [AU: 7] They have found that repeated light touch to an inflamed paw produced cumulative allodynia. Progressive tactile hypersensitivitv can only be induced in inflamed tissue and persists for several hours. It is different from central sensitization induced by C fiber stimulation, which can be induced in noninflamed tissue and lasts only for minutes. Progressive tactile hypersensitivity demonstrates that A-ß afferents have the capacity to produce wind-up of spinal cord neurons, normally a C-fiber mediated effect.
Myofascial pain is not solely A-d and C fiber nociceptive pain. Muscle shortening is an essential component; by simply releasing a shortened muscle, pain is banished. If large diameter A-P primary afferents from the cutaneous nerve can contribute to hyperalgesia, is it possible for large diameter proprioceptor fibers from the muscle nerve to likewise contribute to myofascial pain? Fibers from muscle fascia and other deep tissues must now be studied, in particular group I and II fibers, which sense muscle length and tension, and group III and IV fibers, which sense muscle pain.
In chronic pain, fibrosis eventually becomes a major feature of the contracture; response to dry-needle treatment is then much less dramatic. The extent of fibrosis does not correlate with chronologic age: Scarring can occur after injury or surgery, and many older individuals have sustained less wear and tear than younger ones who have subjected their musculature to repeated physical stress. The treatment of extensive fibrotic contractures necessitates more frequent and extensive needling. To relieve pain in such a muscle, it is necessary to needle all-tender bands. It is uncommon to encounter a muscle that is totally fibrotic and cannot be released by vigorous needling.
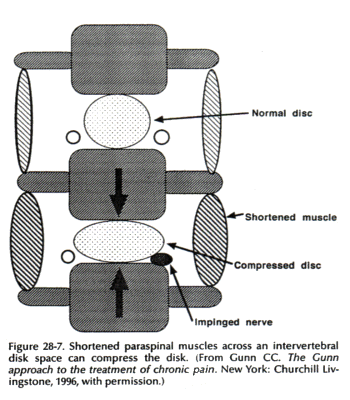
For long-lasting pain relief and restoration of function, it is essential to release shortened paraspinal muscles that may be compressing a disk and disperse fibrotic tissue that may be entrapping a nerve root (Fig. 28-7). Surgical release is rarely necessary as the needle can reach deeply located shortened muscles.
Myofascial pain syndromes frequently become medical riddles when they are not recognized as the effects of neuropathy presenting primarily in the musculoskeletal system. The primary problem is peripheral neuropathy, and pain is just one of its many possible presentations. Pain is not a feature unless nociceptive pathways are involved. Many neuropathic conditions are pain free. It is helpful to remember the following points:
Because pain is a manifestation of neuropathy, therapy should aim at the cause of the neuropathic condition.
Spondylosis is, by far, the most common cause of radiculopathy. Myofascial pain syndromes are almost invariably segmental; symptoms are found in dermatomes, myotomes, and sclerotomes. Examination and treatment must always include the spine.
Establish that neuropathy is present. Signs of neuropathy are subtle and differ from those of outright denervation. Look for vasoconstriction and trophedema.
Neuropathic pain has a proprioceptive component; it cannot exist without muscle shortening. Look for tender, shortened muscles in myotomes.
Neuropathic pain is often the unsuspected cause of many other conditions (e.g., tension headache, frozen shoulder, tennis elbow, and even low back pain). Muscle shortening upsets joint alignment and increases pressure on articular surfaces. Neuropathy degrades the quality of collagen and contributes to degeneration in weight-bearing and activity-stressed parts of the body.
Radiculopathy is perpetuated when shortened paraspinal muscles draw adjacent vertebrae together to compress the disk and irritate the nerve root. The vicious circle must be treated at the spine.
It is only through hands-on examination of patients, explicitly searching for neuropathic signs, that one is able to understand and treat neuropathic myofascial pain. Drug treatment is difficult, and physical therapy is the first approach. The efficacy of intramuscular stimulation for chronic low back pain has been demonstrated by a randomized clinical trial involving a large group of Workers' Compensation Board patients. At their 7-month follow-up, the treated group was clearly and significantly better than the control group (27). It is a most convincing experience to diagnose neuropathic pain by finding its unmistakable physical signs, then to treat the patient with intramuscular stimulation and witness the signs disappear, often within minutes.
Gunn CC. "Prespondylosis" and some pain syndromes following denervation supersensitivity Spine 1978:5:185-192.
Gunn CC, Milbrandt WE. Tenderness at motor points-a diagnostic and prognostic aid for low back injury. J Bone joint Surg 1976; 58A:815-825.
Gunn CC, Milbrandt WE. Tennis elbow and the cervical spine.
Can Med Assoc J 1978;114:803-825.
Gunn CC, Milbrandt, WE. Tenderness at motor points: an aid in the diagnosis of pain in the shoulder referred from the cervical spine. JAOA 1977;77; 196-212.
Gunn CC. The Gunn approach to the treatment of chronic pain-intramuscular stimulation for myofascial pain of radiculopathic origin. 2nd ed. London: Churchill Livingstone,1996.
Thomas PK. Symptomatology and differential diagnosis of peripheral neuropathy: clinical and differential diagnosis. In: Dyck PJ, Thomas PK, Lambert EH, Bunge R, eds. Peripheral neuropathy. Philadelphia: Saunders, 1984:1169-1190.
Cannon WB, Rosenblueth A. The supersensitivity of denervated structures, a law of denervation. New York: MacMillan, 1949.
Sharpless SK. Supersensitivity-like phenomena in the central nervous system. Fed Proc 1975;34:1990-1997.
Culp WJ, Ochoa J. Abnormal nerves and muscles as impulse generators. New York: Oxford University Press, 1982.
Thesleff S, Sellin LC. Denervation supersensitivity. Trends Neurosci 1980;August:122-126.
Sola AE. Treatment of myofascial pain syndromes. In: Benedetti C, Chapman CR, Morrica C, eds. Advances in pain research and therapy. New York: Raven, 1984;7:467-485.
Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain 1977,3:25-11.
Gunn CC, Milbrandt WE. Early and subtle signs in low back sprain. Spine 1978;3:267-281.
Klein L, Dawson MH, Heiple KG. Turnover of collagen in the adult rat after denervation. J Bone joint Surg 1977;59A:1065-1067.
Wall PD, Gutnick M. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol 1974;43;580-593
Govrin-Lippmann R, Devor M. Ongoing activity in severed
nerves:
source and variation with time. Brain Res 1978;159:406-410.
Devor M. Pain mechanisms. Neuroscience 1996;2:233-244.
Munglani R, Hunt SP, Jones JG. The spinal cord and chronic
pain. In: Kaufman L, Ginsburg R, eds. Anaesthesia review. New York:
Churchill Livingstone, 1996;12:53-76.
Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain 1993;52:259-285.
Coderre TJ. The role of excitatory ammo acid receptors and intracellular messengers in persistent nociception after tissue injury in rats. Mol Neurobiol 1993:7:229-246.
Devor M, Rappaport HZ. Pain and the pathophysiology of damaged nerve. In: Wall PD, Melzack R, eds. Textbook of pain. Edinburgh: Churchill Livingstone, 1989:47-83.
Woolf CJ, Doubell TP. The pathophysiology of chronic pain - increased sensitivity to low threshold AB-fiber inputs. Curr Opin Neurobiol 1994,4:525-534.
Woolf CJ, Thompson S\VN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991:44:293-299.
Gunn CC. The mechanical manifestations of neuropathic pain. Ann Sports Med 1990;5:138-141.
Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain 1997;73:123-139.
Lomo T. The role of activity in the control of membrane and contractile properties of skeletal muscle In: Thesleff S, ed. Motor innervation of muscle. New York: Acadentic Press, 1976:289-316
Gunn CC, Milbrandt WE. Dry needling of muscle motor points for chronic low back pain. A randomized clinical trial with long-term follow-up. Spine 1980;5:279-291
Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain 1977;73:87-95.
Fields HL. Pain. New York: McGraw-Hill, 1987:133-169.
© iSTOP - Institute for the Study and Treatment of Pain - 2002